Bedrock Digital Solutions
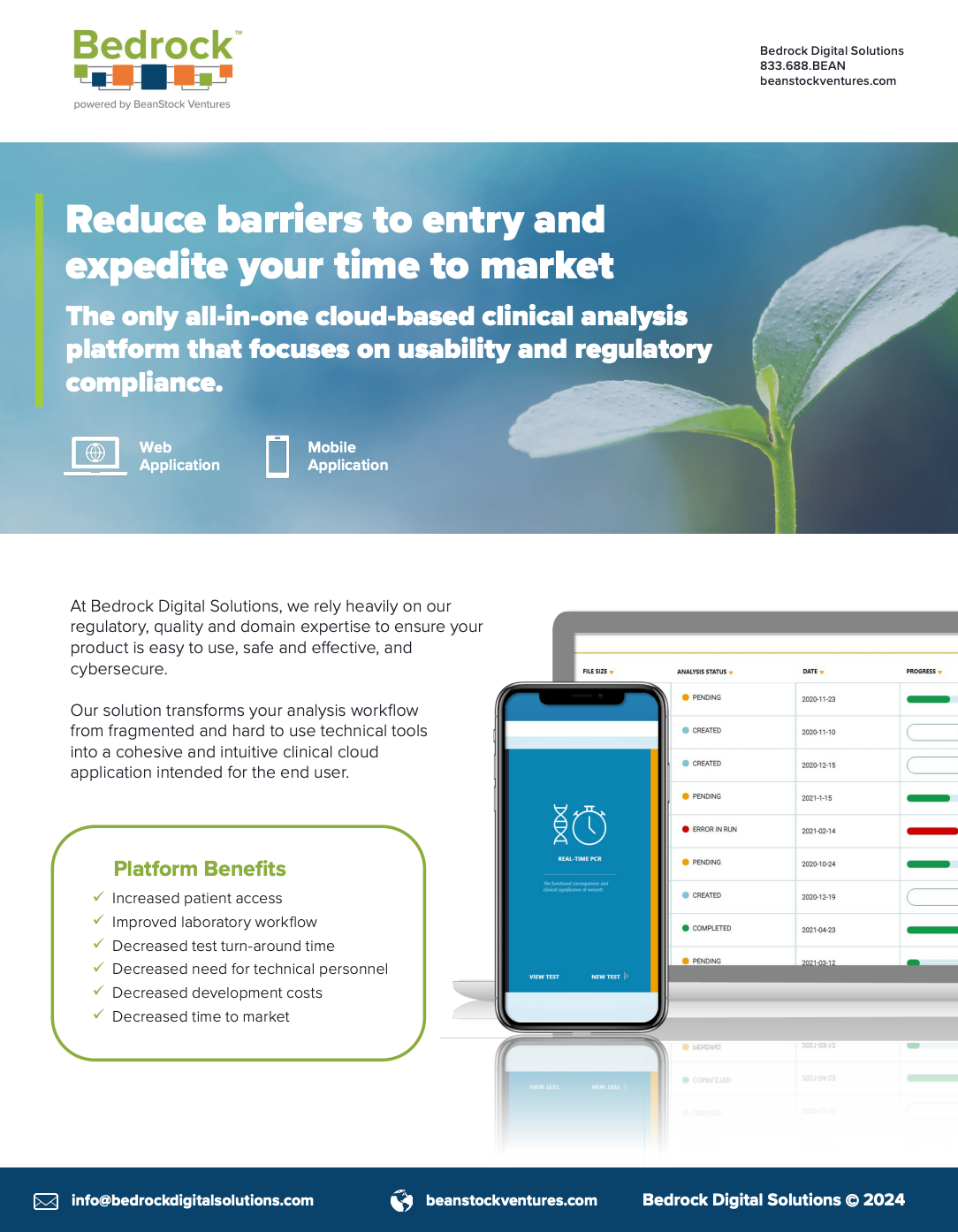
Reduce barrier to entry and expedite your time to market
What is Bedrock?
Bedrock™ is the only all-in-one SaaS, cloud-based clinical analysis platform designed for the healthcare and diagnostics industry, delivering unmatched usability, seamless workflow integration, and full FDA, Cybersecurity, HIPAA, and GDPR compliance—helping you launch faster, safer, and smarter.
Bedrock allows you to:
Accelerate time-to-market with a pre-validated, regulatory-compliant software platform built for medical device software and clinical diagnostics.
Integrate effortlessly with EMR, LIMS, and patient/physician portals for secure, automated data and analysis management.
Reduce costs and compliance risk through built-in cybersecurity, continuous updates, and expert regulatory alignment.
What’s included
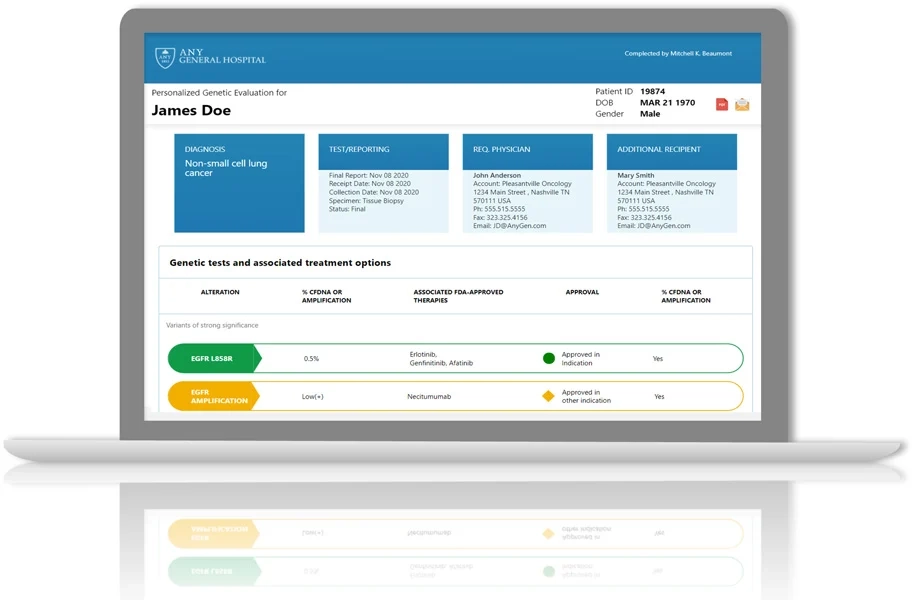
A clinical grade regulatory complaint custom application with a powerful clinical analysis platform.

Access Bedrock Clinical Analysis Platform
Harness Bedrock’s secure, cloud-based clinical analysis platform with intuitive dashboards and a robust data management backend. Gain FDA-ready compliance and customer confidence, advanced cybersecurity, and seamless EMR/LIMS integration—streamlining workflows, accelerating development and approvals, and reducing costs.

Stay Informed, Supported, and Connected
Stay ahead with mobile app access and platform independence, ensuring your customers can securely connect from anywhere. Get ongoing platform enhancements, compliance updates and expert support from our software and deployment specialists—plus access to a new features.
Customize and Scale with Confidence
Create custom analysis tools and workflows tailored to your diagnostics and clinical needs. Integrate advanced analytics, adapt for new markets, and expand capabilities—while we handle all software maintenance, updates, security patches, and regulatory compliance in the background.
-
Featured Case Study
From Submission to Clearance in 60 Days: How Medical Templates Fast-Tracked FDA Approval with 3P510(k)
Read the full story: From Submission to Clearance in 60 Days: How Medical Templates Fast-Tracked FDA Approval with 3P510(k)Summary Medical Templates AG, a Swiss-based MedTech innovator, had a strong internal team, an established QMS, and a well-prepared FDA submission package for its image-guided software system, Puncture Cube. Facing tight timelines due to an upcoming trade show, the company partnered with BeanStock Ventures to expedite clearance. By leveraging our FDA-accredited 3P510(k) review pathway and…

Fast-track your SaMD product with an all-in-one clinical analysis platform
Bedrock transforms fragmented software analysis tools into a cybersecure, intuitive, and regulatory-compliant cloud platform—helping you reduce time to market, lower development costs, and meet FDA and IEC 62304 requirements with confidence
Expert Help From Start to Finish
A proven system to accelerate your time to market
Regulatory Strategy
Just beginning, or require clarity on your regulatory pathway? Start with our foundational regulatory strategy services to chart the most effective course to market readiness.
QMS & Technical Documentation Support
Ensure your QMS is in place and your DHF is complete with services like audits, assessments, design reviews, and documentation creation or updates.
FDA Submission Support
Navigate the entire FDA submission process with clear guidance on Q-Submission, eStar review or assembly, FDA additional information requests, or deficiencies.
Post-Market Support
After approval, get support with company and product registrations and ongoing compliance so your product stays current with FDA requirements.
Let’s Build Your Path
to Approval
We’ll work with you to develop a clear plan that keeps things moving, helps you avoid delays, and gets your product ready for FDA approval.
We Have the Answers to
Your Top Questions
How can Bedrock help if I have limited understanding of software regulatory requirements?
Bedrock™ if development under a ISO 13485 compliant Quality Management System and adheres to FDA, IEC 62304, and SaMD regulatory standards—covering cybersecurity, data privacy, AI compliance, and risk management. This eliminates the need to become a regulatory expert, allowing you to focus on innovation while we handle compliance.
My current clinical or research analytical solution is hard to use. Can Bedrock fix this?
Absolutely. Bedrock’s platform is FDA-ready and supports every stage of Software as a Medical Device (SaMD) approval, from basic to enhanced classifications. We streamline documentation, testing, and submission to reduce approval timelines and speed your time to market.
I have a clinical analysis algorithm that needs FDA approval—can Bedrock support this process?
At the end of the 12 months, you will have your DHF free and clear and can renew your training annually to meet FDA requirements.
What if I have limited software engineering resources but need to launch quickly?
Bedrock’s cloud-based, turnkey architecture reduces development costs and eliminates the need for large engineering teams. With pre-built integrations, customizable workflows, and automated maintenance, you can launch faster—often cutting time-to-market significantly while ensuring regulatory compliance.
Don’t see what you’re looking for? Reach out to our team