3P510(k) Review
– Skip the Line to FDA Clearance
Cut approval times by over a year and bring your Class II device to market in as little as 60 days
What is FDA’s Third-Party Review Program?
The 510(k) Third Party Review Program provides medical device manufacturers with a voluntary alternative review process, in which accredited Third Party Review Organizations (3P510k Review Organizations), such as BeanStock Consulting, are allowed to review select medical devices while still maintaining FDA oversight.
The FDA Third-Party Review Program allows you to:
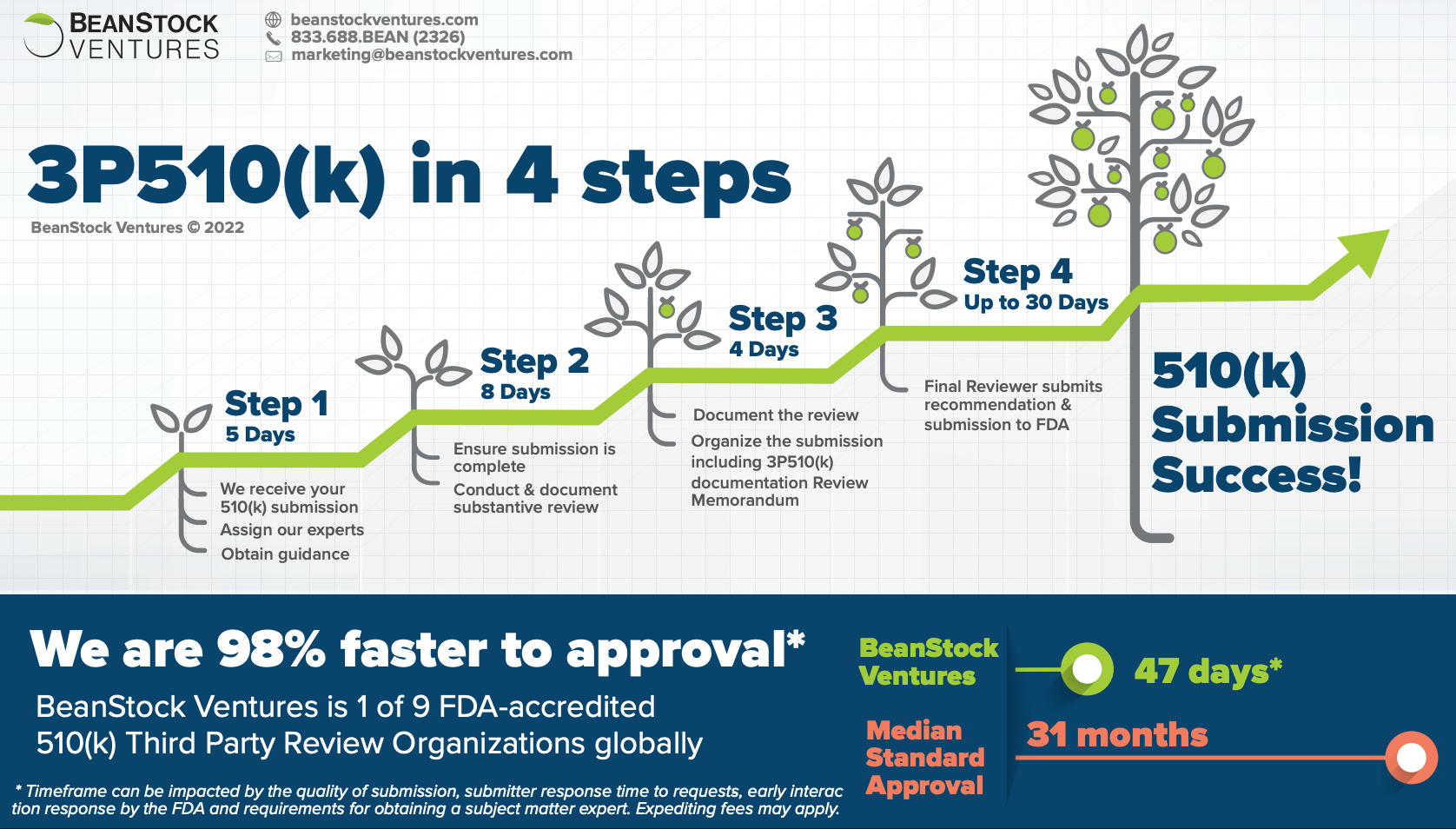
Fast-track your clearance by getting FDA review and recommendation in as little as 60 days, compared to the 31-month median for traditional submissions.
Save time and resources by working with 1 of only 7 FDA-recognized review organizations worldwide, experienced in over 250 Class II product codes.
Reduce regulatory uncertainty with early FDA-equivalent feedback with a dedicated reviewer that minimizes delays.
What’s the process
We make the 3P510(k) process simple—early interactions with the FDA, conducting the FDA-equivalent review,
providing comprehensive requests for information, and delivering a recommendation to and obtaining a
response from the FDA in as little as 60 days.
Learn more about the FDA 3P510K program
Discover how the FDA’s Third-Party Review Program can dramatically shorten clearance times for eligible Class II devices by leveraging accredited reviewers like BeanStock Consulting to perform FDA-equivalent reviews.
Determine if your device qualifies
Find out whether your device’s classification and product code are eligible for Third-Party review—roughly 50% of Class II product codes currently qualify, including many in-vitro diagnostics and digital health technologies.
Understand the submission process
See how the 3P510(k) pathway works—from initial eligibility review through submission assembly, third-party evaluation, and final FDA clearance recommendation.

BeanStock Ventures (BSV) declares that it protects against the use, in carrying out any review of a 510(k) submission and initial classification of a device of any officer or employee and maintains compliance with requirements relating to financial conflicts of interest.
-
Featured Case Study
From Submission to Clearance in 60 Days: How Medical Templates Fast-Tracked FDA Approval with 3P510(k)
Read the full story: From Submission to Clearance in 60 Days: How Medical Templates Fast-Tracked FDA Approval with 3P510(k)Summary Medical Templates AG, a Swiss-based MedTech innovator, had a strong internal team, an established QMS, and a well-prepared FDA submission package for its image-guided software system, Puncture Cube. Facing tight timelines due to an upcoming trade show, the company partnered with BeanStock Ventures to expedite clearance. By leveraging our FDA-accredited 3P510(k) review pathway and…

Clear your device in as little as 60 days
As 1 of only 7 FDA-accredited third-party review organizations, our 3P510(k) service helps you bypass traditional FDA delays, offering a faster route to market with expert-reviewed FDA approved submissions for over 250 product codes
Expert Help From Start to Finish
A proven system to accelerate your time to market
Regulatory Strategy
Just beginning, or require clarity on your regulatory pathway? Start with our foundational regulatory strategy services to chart the most effective course to market readiness.
QMS & Technical Documentation Support
Ensure your QMS is in place and your DHF is complete with services like audits, assessments, design reviews, and documentation creation or updates.
FDA Submission Support
Navigate the entire FDA submission process with clear guidance on Q-Submission, eStar review or assembly, FDA additional information requests, or deficiencies.
Post-Market Support
After approval, get support with company and product registrations and ongoing compliance so your product stays current with FDA requirements.
Let’s Build Your Path
to Approval
We’ll work with you to develop a clear plan that keeps things moving, helps you avoid delays, and gets your product ready for FDA approval.
We Have the Answers to
Your Top Questions
What is the FDA Third-Party Review Program?
It’s an FDA initiative that allows accredited organizations like BeanStock Consulting to review certain Class II medical devices and submit clearance recommendations directly to the FDA, significantly reducing the review timeline.
How much faster is the 3P510(k) process compared to traditional review?
Traditional FDA reviews can take over 31 months, but with BeanStock Consulting as your third-party reviewer, most eligible devices achieve clearance in as little as 60 days.
How do I know if my device qualifies?
The FDA maintains a list of hundreds of Class II product codes eligible for Third-Party review. BeanStock Consulting can quickly assess your product’s classification and eligibility during an initial free-consultation.
Is the review process different from a standard FDA review?
The requirements and rigor are the same—the difference is speed. BeanStock Consulting allocates dedicated resources during the lifetime of your review and performs an FDA-equivalent review, raises issues quickly, and delivers a clearance recommendation directly to the FDA for consideration, reducing back-and-forth and delays.
Don’t see what you’re looking for? Reach out to our team