The 510(k) Third Party Review Program provides medical device manufacturers with a voluntary alternative review process, in which accredited Third Party Review Organizations (3P510k Review Organizations), such as BeanStock Consulting, are allowed to review select medical devices while still maintaining FDA oversight.
BeanStock Ventures (BSV) declares that it protects against the use, in carrying out any review of a 510(k) submission and initial classification of a device of any officer or employee and maintains compliance with requirements relating to financial conflicts of interest.

BeanStock Ventures receives Third Party Review 510(k) Inquiry.

BeanStock Ventures schedules a call to describe the process, answer any questions and to discuss timing and pricing.

BeanStock Ventures determines if the medical device is eligible for their review.
If the medical device is eligible and the client wishes to proceed:

BeanStock Ventures sends a mutual non-disclosure agreement to the interested party with signing authority.

BeanStock Ventures sends a contract to the interested party with signing authority with cost estimate and retainer cost to reserve their requested submission dates.
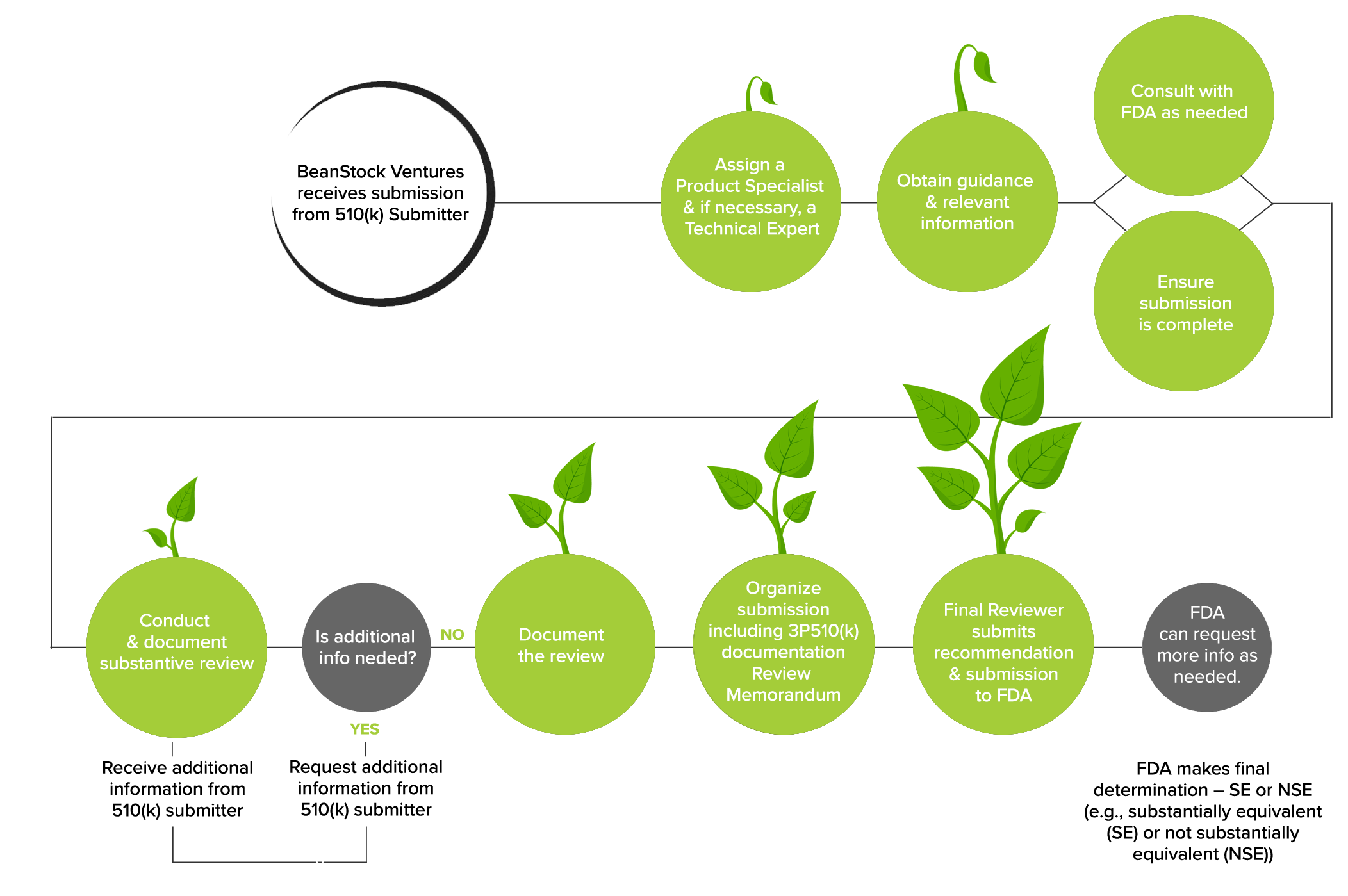
Once the contract is executed, and the retainer is received, the process defined below starts:
© 2024 BeanStock Ventures